A guide to supercritical CO2 fluid extract in 2022
What is supercritical fluid extraction method?
Supercritical fluid extraction (SFE) is a method used to extract desired compounds from a variety of materials using a supercritical fluid as the solvent. Supercritical fluids are substances that are above their critical temperature and critical pressure, where they exhibit properties of both a liquid and a gas. The most commonly used supercritical fluid for extraction is carbon dioxide (CO2) due to its favorable characteristics.
Top 6 Steps of Supercritical Fluid Extraction Method
The supercritical fluid extraction method involves the following steps:
Steps 1#: Selection of the Supercritical Fluid
The choice of the supercritical fluid depends on factors such as the nature of the target compounds, operating conditions, and safety considerations. CO2 is frequently chosen due to its low toxicity, availability, and mild critical parameters.
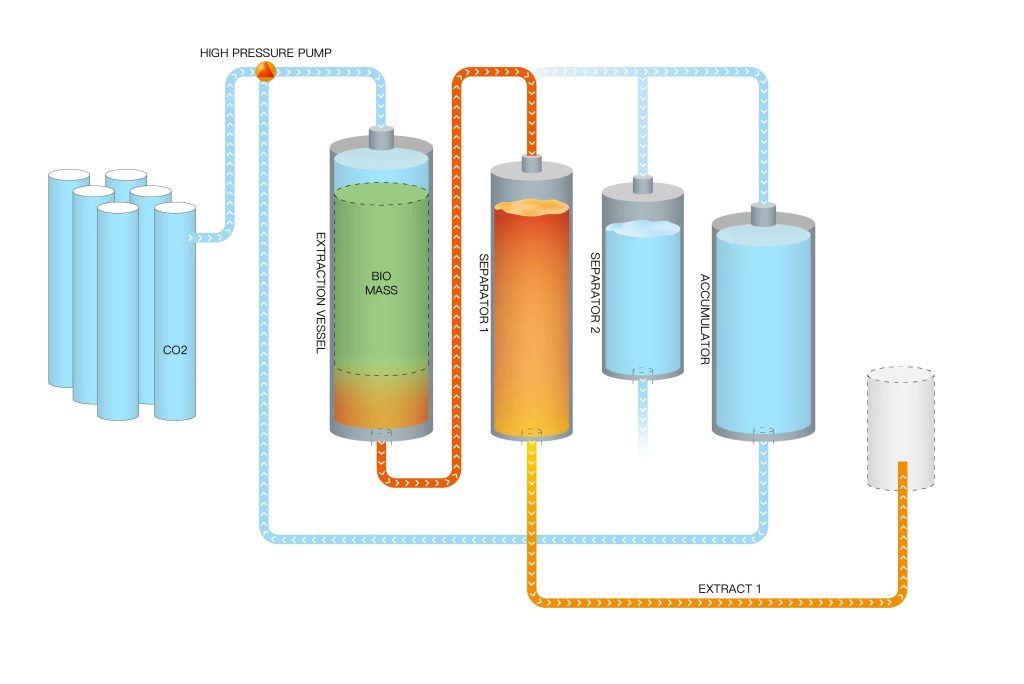
Steps 2#: System Setup
The extraction system consists of a high-pressure vessel, a pump, a heat exchanger, and a separator. The high-pressure vessel is designed to withstand the high pressures required for creating the supercritical state of the fluid. The pump circulates the supercritical fluid through the system, while the heat exchanger helps control the temperature. The separator is used to separate the extracted compounds from the supercritical fluid.
Steps 3#: Adjustment of Parameters
The extraction parameters, including temperature, pressure, and flow rate, are adjusted to optimize the extraction process. These parameters are specific to the material being extracted and the desired compounds.
Steps 4#: Loading the Material
The material containing the target compounds is loaded into the extraction vessel. This can be in the form of solid particles, liquid solutions, or even gases.
Steps 5#: Extraction
The supercritical fluid is compressed and heated to reach the supercritical state. In this state, the fluid exhibits properties of both a liquid and a gas, allowing it to penetrate the material and dissolve the target compounds. The solubility of compounds in the supercritical fluid depends on the operating conditions and the nature of the compounds.
Steps 6#: Separation
After extraction, the mixture of supercritical fluid and dissolved compounds is directed to the separator. Here, the pressure is reduced, causing the supercritical fluid to revert to its gas phase. The target compounds, which are no longer soluble in the gas phase, separate from the supercritical fluid as a concentrated extract.
Supercritical fluid extraction offers several advantages over traditional extraction methods. It is a selective process that allows for the extraction of specific compounds while leaving undesirable components behind. Additionally, it operates at relatively low temperatures, reducing the risk of thermal degradation. The use of supercritical CO2 as the solvent eliminates the need for toxic or flammable solvents, making it an environmentally friendly option.
Supercritical fluid extraction has found applications in various industries, including food and beverage, pharmaceuticals, cosmetics, and natural product extraction. It is used to extract a wide range of compounds, such as essential oils, flavors, fragrances, pharmaceutical actives, and environmental contaminants.
Overall, supercritical fluid extraction is a versatile and efficient method for extracting desired compounds, offering numerous benefits and applications across different sectors.
What is supercritical CO2 fluid?
In SFE technology, supercritical CO2 extraction (SC-CO2) using CO2 as the extraction medium is the most widely used.
The most commonly used supercritical fluid is supercritical carbon dioxide because it has moderate critical temperature ( 31.26℃ ) and pressure (72.9 atm.).
Why is carbon dioxide used as a supercritical fluid?

The properties of carbon dioxide will change when the temperature is higher than the critical temperature Tc=31.26℃ and the pressure is higher than the critical pressure Pc=72.9atm. Its density is close to liquid, its viscosity is close to gas, and its diffusion coefficient is 100 times that of liquid. It has amazing dissolving power.
Furthermore, because carbon dioxide is a gas at room temperature, it can easily be separated/removed to yield a solvent-free extract.
Supercritical carbon dioxide can dissolve a variety of substances, and then extract the effective ingredients, which has a wide range of application prospects.
Supercritical carbon dioxide is one of the most widely studied fluids because it has the following characteristics:
- The critical temperature of CO2 is 31.26℃, the critical pressure is 72.9atm, and the critical conditions are easily reached.
- CO2 is chemically inactive, colorless, odorless, non-toxic, and safe.
- The price is cheap, the purity is high, and it is easy to obtain.
Is supercritical CO2 extraction (SFCE) safe?
As far as non-polar solvents are concerned, CO2 is among the safest. In fact, the FDA has labelled CO2 safe for industrial extractions, making it a much less controversial solvent than petroleum based hydrocarbons such as butane or propane.
Is supercritical CO2 extraction better?
Supercritical CO2 extraction is also much better for the environment. CO2 gas is natural, so if it escapes into the air during extraction it won’t do any harm. It can also be recycled, making this method much more sustainable. Since the solvent isn’t toxic, people working with this extraction method don’t face any health risks.
Is supercritical CO2 corrosive?
Dry supercritical CO2 fluid is non-corrosive.
Supercritical water fluid is very corrosive.
What is supercritical water?
The critical temperature of water T=374℃, and the critical pressure P=22.1MPa.
When the temperature and pressure of the system exceed the critical point, it is called supercritical water.
What does supercritical water look like?
There is no difference between the liquid and gas of water in the supercritical state, and they are completely blended together to become a new fluid that presents a high-pressure and high-temperature state.
“Supercritical” can be considered the “fourth state” of the material. It is not a solid, liquid or gas-it is in the form of steam.
What are the characteristics of supercritical water?
It has a strong oxidizing ability. Put the material to be treated into supercritical water, and then dissolve oxygen in it (which can be dissolved in a large amount). Its oxidizing ability is stronger than potassium permanganate.
- Many substances can burn in it and emit flames.
- It can dissolve many substances (such as oil), and the volume will be greatly reduced when dissolved, because the supercritical water will tightly wrap the oil at this time.
- It can slowly dissolve and corrode almost all metals, even gold (similar to aqua regia).
- Super catalysis, in supercritical water, chemical substances will react very quickly, and some can reach 100 times the horror!
What can supercritical CO2 extract?
Supercritical fluid extraction with CO2 as a single medium is mainly used for lipophilic, non-polar substances with small molecular weight, that is, the s
Supercritical fluid extraction with CO2 as a single medium is mainly used for lipophilic, non-polar substances with small molecular weight.
That is, the compounds mentioned in the solubility rule ② and the solubility rule ③ that are miscible with supercritical CO2 or have high solubility.
If the components contained in the raw materials belong to the compounds mentioned in the solubility rule ⑤ and the solubility rule ⑥, they are difficult to extract and remain in the raffinate.
What can supercritical CO2 dissolve?
Studies have shown that the extracts of supercritical pure CO2 are mostly a mixture of volatile oils, fats, alcohols, ethers, esters, resins and other lipophilic chemical components.
What are the advantages and disadvantages of supercritical fluid extraction?
What are the advantages of supercritical CO2 (SFE)?
What are the benefits of using supercritical fluids?
Supercritical carbon dioxide is used in large quantities for extraction because it has the following characteristics of extraction technology:
Natural and environmentally friendly, no soluble residue
Supercritical CO2 fluid is a colorless, odorless and non-toxic gas under normal conditions. After separation from the extracted components, there is no solvent residue at all, which can effectively avoid solvent toxicity residues under traditional solvent extraction conditions.
At the same time, it also prevents the extraction process from toxic to the human body and pollution to the environment. It is a natural and environmentally friendly extraction technology.
Extract near room temperature
Since supercritical CO2 extraction can achieve supercritical extraction near room temperature, it can avoid the decomposition of heat-sensitive substances caused by high-temperature operation, and the solute separation is easy to have the advantages of no solvent residue problems, which is beneficial to ensure and improve the quality of natural products, so , Supercritical CO2 extraction is regarded as a green chemical technology.
Why is CO2 extraction better than steam distillation?
In steam distillation, the molecular composition of both the plant matter and the essential oil are changed due to the temperature applied. On the other hand, a CO2 extract is closer in chemical composition to the original plant from which it is derived, as it contains a wider range of the plant’s constituents.
Completely retain the biological activity of biological objects
The extraction temperature is low, the critical temperature of CO2 is 31.265℃, and the critical pressure is 72.9atm, which can effectively prevent the oxidation, dissipation and reaction of heat-sensitive components, and completely retain the biological activity of biomass objects;
At the same time, high boiling point, low volatility, easily pyrolyzable substances can be extracted below its boiling temperature.
High extraction efficiency and low energy consumption
Extraction and separation are combined into one. When the supercritical fluid of carbon dioxide saturated with dissolved matter flows through the separator, the pressure drop causes the CO2 and the extract to quickly return to two separate phases (gas-liquid separation) and separate immediately, and there is no material. The phase change process does not require solvent recovery and is easy to operate;
It not only has high extraction efficiency, but also consumes less energy, saves costs, and conforms to the trend of environmental protection and energy saving.
Easy to operate, less time-consuming, small area, friendly to the environment
The extraction operation is easy, and both pressure and temperature can be used as parameters for adjusting the extraction process. Near the critical point, small changes in temperature and pressure will cause significant changes in CO2 density, which will cause changes in the solubility of the substance to be extracted. This can be achieved by controlling the temperature or The pressure method achieves the purpose of extraction. The pressure is fixed, and the material can be separated by changing the temperature;
Is CO2 extraction healthy?
The use of carbon dioxide provides a cleaner, healthier product.
Unlike solvents such as butane and hexane, CO2 extraction contains no solvents or chemical residues. It is also recyclable and better in the long run for the planet.
On the contrary, the temperature is fixed and the pressure is reduced to separate the extracts; therefore, the technical process is short, time-consuming, and small in area. At the same time, it is truly environmentally friendly. The extraction fluid CO2 can be recycled and will not emit waste carbon dioxide to cause the greenhouse effect! Become a truly “green” production process.
Polarity can be changed
The polarity of the supercritical fluid can be changed. Under certain temperature conditions, as long as the pressure is changed or a suitable entrainer is added, substances of different polarities can be extracted, and a wide range of options are available.
What is the main disadvantage of supercritical fluid extraction?
- The introduction of strong polar groups (-OH, -COOH) makes extraction difficult.
Within the scope of benzene derivatives, substances with three hydroxyphenols and compounds with one carboxyl group and two hydroxyl groups can still be extracted, but those with one carbonyl group and more than three hydroxyl groups cannot be extracted. - Stronger polar substances, such as sugars, are difficult to extract below 40MPa.
- The higher the relative molecular mass of the compound, the more difficult it is to extract.
- The main disadvantage of supercritical carbon dioxide extraction is the expensive equipment and the analysis process.
The main disadvantage of SFE is that the extraction must be performed at the high pressure (100-1,000 bar) required to maintain the solvent in a supercritical state. This means that the processing time is shortened, that is, more effective ingredients can be produced in a smoother and gentler way. This creates greater commercial use value. The result is higher capital and operating costs.
Is CO2 extraction better than cold pressed?
Supercritical CO2 extraction is optimization because oxygen-free environments and low temperatures are particularly important when extracting fragile oils like plant essentical oil.
By keeping temperatures low and extracting all the ‘active’ components, supercritical co2 extraction able to preserve and protect the complete botanical, skin-loving properties of the plant essentical oil.
For example, SC-CO2 process delivers twice the regenerative sterols and five times more of those carotenoids than your average Rosehip seed oil. It also means it lasts four times longer in your bathroom cabinet!
CO2 extraction also leaves absolutely no solvent residues, so the final plant essentical oil is impeccably pure. The low temperature and lack of waste streams/emissions make it an extremely environmentally friendly process, too!
What is the main advantage of using supercritical fluid over regular fluid for extraction process?
7 advantages of supercritical CO2 fluid extraction
The advantages of using SFE when compared to conventional methods are:
- Higher selectivity because the solvation power of the fluid can be adjusted by changing temperature and pressure;
- Lower viscosity and higher diffusivity of supercritical fluids allow faster mass transfer of solutes from porous plant materials;
- SFE can be performed at low temperatures making the process ideal for the extraction of thermally labile compounds;
- Extracts dissolved in supercritical carbon dioxide can be separated by depressurization with little to no solvent residue left behind;
- SFE units can be coupled to a GC–MS or NMR allowing extraction, analysis, and quantification of extracted molecules instantaneously;
- SFE can be carried out at different scales: analytical (less than 1 g sample), preparative (hundreds of grams of sample), pilot (kilograms of sample), and industrial (tons of sample).
- Finally, at industrial scale, supercritical carbon dioxide is normally reused, therefore minimizing waste generation.
Comparison of SFE and traditional methods
The supercritical CO2 extraction of clove oil was also compared with several traditional methods.
The results showed that the oil recovery rate of SFE reached 19.6% (mass fraction), which was nearly double the yield of steam distillation and water distillation;
The main active substance phenols (eugenol + aceteugenol) content is also the highest (78.4%, mass fraction);
At the same time, the extraction time of SFE is short (2h), there is no solvent residue, and the product is a high-quality light yellow oil.
Water distillation
The darker color of the essential oils distilled in water indicates that the pyrolysis is serious, the oil yield is low, and there are solvent residues.
Soxhlet
When absolute ethanol is used as the solvent, the extract obtained by Soxhlet extraction is brown extract, it is difficult to obtain light yellow clove oil, and post-processing is necessary.
The results show that the supercritical CO2 extraction technology of clove oil is far superior to other extraction methods.
3 minutes of professional knowledge reading
Solubility of solute in supercritical carbon dioxide
Studying the solubility of solutes in supercritical CO2 has theoretical and practical application significance. The CO2 solubility rules summarized by the research results of Francisi, Stahl, Hyatt and other scholars,
It is helpful for people to initially understand the solubility and selectivity of various solutes in supercritical CO2. The solubility rules are as follows
Rule 1
The solubility value of solute in subcritical CO2 and supercritical CO2 generally differs by about an order of magnitude, but it has never been found that any kind of substance does not dissolve in subcritical CO2 but dissolves in a supercritical state, indicating that the substance is in subcritical CO2. The solubility behavior of CO2 and supercritical CO2 is continuous
Rule 2
CO2 has a strong homogenization effect. Studies have shown that at least 140 compounds can form a homogeneous miscible state with CO2 at moderate pressure and room temperature, that is, liquid CO2 and supercritical CO2 can be miscible with many non-polar and weakly polar solutes, such as carbon Normal alkanes with less than 12 atoms, normal alkenes with less than 10 carbon atoms, lower alcohols with less than 6 carbon atoms in the main chain, and lower fatty acids with less than 10 carbon atoms in the main chain. Ester compounds that are equal to 12 and the number of carbon atoms in the alcohol backbone is less than or equal to 4, low-carbon aldehydes with carbon atoms less than 7, low-carbon ketones with carbon atoms less than 8, low-carbon ethers with carbon atoms less than 4, etc.
Rule 3
Although liquid CO2 and supercritical CO2 under medium pressure have excellent solubility for the aforementioned aliphatic hydrocarbons and low-polar lipophilic compounds, as the number of carbon atoms increases, that is, with the increase of the chain length and molecular weight With the increase of, its solubility in liquid CO2 and supercritical CO2 will change from miscible state to partial solubility, and the solubility will gradually decrease
Rule 4
Strong polar functional groups (such as the introduction of O11COD will reduce the solubility of compounds, so polyols, polyacids, and multiple hydroxyl and carboxy And the solubility in supercritical CO2 is extremely low.
Rule 5
Liquid CO2 and supercritical CO2 are suitable for most mineral inorganic salts and highly polar substances (such as sugars, amino acids, starches, proteins, etc.) are almost insoluble, so they will not be extracted during sub-supercritical and supercritical CO2 extraction processes
Rule 6
Liquid CO2 and supercritical CO2 are almost insoluble to compounds with a molecular weight of more than 500
Summary
The above rules provide a reference for the preliminary qualitative judgment of whether a substance can use supercritical CO2 as the extraction solvent, and also provide a basis for the range of substances that can be selected as a co-reagent when a co-reagent (cden) needs to be added in the CO2 extraction process.