The basic principle of supercritical CO2 fluid extraction technology
Supercritical fluid to be sensitive to pressure and temperature changes, and use the supercritical fluid (such as SC-CO2) as the separation medium (extractant), and the fluid has a high degree of enhancement in the supercritical state.
Utilizing the relationship between the dissolving ability of supercritical fluid and its density, that is, using the influence of pressure and temperature on the dissolving ability of supercritical fluid. In the supercritical state, the supercritical fluid is contacted with the substance to be separated to selectively extract the components of polarity, boiling point, and molecular weight in sequence.
Extractant is reduced by continuous adjustment of temperature and pressure, thereby reducing its solubility to the solute and making the solvent It is effectively separated from the solute (extract).
Of course, it is impossible to obtain a single extract corresponding to each pressure range, but the conditions can be controlled to obtain the optimization ratio of the mixed components, and then the supercritical fluid can be turned into an ordinary gas by means of decompression and temperature increase, and the extracted substance is It is completely or basically precipitated to achieve the purpose of separation and purification, so the supercritical CO2 fluid extraction process is a combination of extraction and separation processes.
What are the advantages of supercritical CO2 extraction?
Supercritical CO2 extraction principle VS traditional extraction principle
In the traditional separation method, solvent extraction uses the difference in affinity (shown in solubility) between the solvent and each solute to achieve separation;
Distillation uses the difference in the volatility (vapor pressure) of the components in the solution to achieve separation.
The supercritical CO2 extraction is separated by adjusting the pressure and temperature of CO2 to control the two parameters of solubility and volatility. Therefore, supercritical CO2 extraction combines the two functions and characteristics of solvent extraction and distillation.
What are the featuress of supercritical CO2 extraction?
- By adjusting the pressure and temperature, it is convenient to change the properties of the solvent and control its selectivity;
- Low temperature extraction, suitable for heat-sensitive substances;
- Supercritical CO2 has low viscosity, large diffusion coefficient and fast extraction speed;
- The separation of solute and solvent is thorough and easy.
How does supercritical fluid extraction work?

The figure below is a schematic diagram of the most basic supercritical extraction process. First, the solvent (such as CO2) is passed through the booster 1 (high-pressure pump or compressor) to reach the supercritical state;

1-Pressure booster; 2,6-heat exchanger; 3-extractor; 4-pressure-reducing valve; 5-separator.
E: Extraction state S: Separation state
Then the supercritical fluid enters the extractor 3 to contact the pre-loaded raw materials (e.g., solid raw materials crushed into a certain particle size) and extract the target solute therein under the conditions of supercritical temperature T1 and pressure p1;

The extract (solute) dissolved in the supercritical fluid is throttled and expanded by the pressure reducing valve 4 as the supercritical fluid leaves from the top of the extractor, so that the density of the supercritical fluid is significantly reduced, thereby reducing the extract in the fluid. The solubility of the extract and the solvent can be separated in the separator 5.
Then make the solvent into a gaseous or supercritical state (the fluid state depends on T2, p2) and leave the separator 5, and then pressurize it to the supercritical state (T1, p1) by the booster 1, and repeat the above-mentioned extraction-separation step, After multiple cycles of the fluid, the extraction effect of the supercritical fluid on the solute reaches the expected value.

The extract deposited in the separator 5 generally enters the collection container through a discharge valve at the bottom of the separator.
Three minutes to read
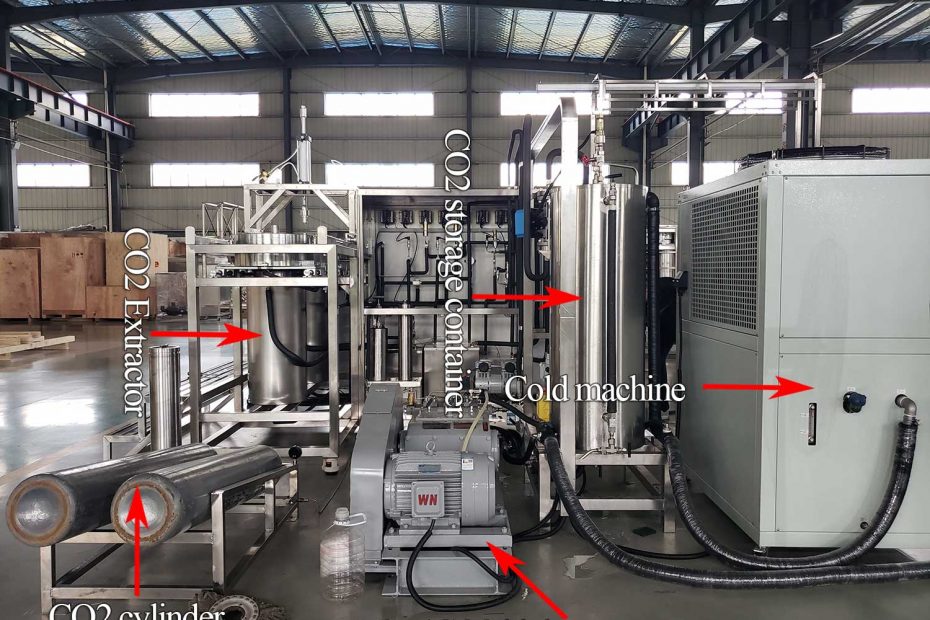
Supercritical fluid extraction system
At present, the large-scale equipment of supercritical extraction process mostly uses high-pressure pumps, and the supercritical extraction of solid materials mostly adopts batch operation; in order to realize a semi-continuous process, several extractors can be operated in parallel, such as three extractors in parallel, when one operation During operation, the other two can be loaded and unloaded separately, and for liquid materials, a continuous co-current (or counter-current) extraction process can be used.
The material system involved in the supercritical extraction process includes the raw material mixture), the benzene extract (the solute) and the extraction medium (single supercritical fluid or mixed supercritical fluid), which constitute a multi-element system.
Solute extracted by supercritical fluid
The vapor pressure, polarity and molecular weight of the solute are important factors that affect the solubility of the solute in the supercritical fluid.
Therefore, the solubility of the solute in the supercritical fluid is not only related to the density of the fluid, but also directly related to the affinity and volatility between the supercritical fluid molecules and the solute molecules, and the difference in volatility between the separated substances during the extraction process These two factors work together with the difference in their intermolecular affinity; for example, in the supercritical extraction process, substances with low boiling points are extracted first than substances with high boiling points. Non-polar supercritical carbon dioxide only affects non-polar and weak substances. Polar substances have a high extraction capacity.
In the chemical unit operation, rectification uses the difference in the volatility of each component to achieve the purpose of separation, and liquid-liquid extraction uses the difference in solubility between the extractant and the molecule to be extracted to separate the extracted components from the mixture.
Because supercritical fluid has excellent mass transfer characteristics of gas and solvation ability equivalent to liquid solvents, supercritical extraction with it as a separation medium is considered to be a combination of distillation and liquid-liquid extraction to a certain extent. The advantages of this form a unique separation technology.
Because this separation process requires auxiliary agents (extractants), most scholars believe that SFE is closer to liquid benzene extraction and solid leaching, which is an extension and expansion of the classic extraction process. Its theoretical basis is that the fluid mixture is in a supercritical state. The phase balance relationship, and its operation belongs to the mass transfer process.
The Principle of Supercritical Fluid Extraction: Harnessing the Power of Supercritical Fluids for Efficient Extraction
Supercritical fluid extraction (SFE) is a powerful technique used to extract desired compounds from a wide range of materials. It relies on the unique properties of supercritical fluids, which exhibit characteristics of both gases and liquids, to efficiently extract target compounds. This article explores the principle behind supercritical fluid extraction, delving into the properties of supercritical fluids, the extraction process, and the advantages it offers over conventional extraction methods.
Properties of Supercritical Fluids
Supercritical fluids are substances that are above their critical temperature and critical pressure, allowing them to exist in a state that combines the properties of gases and liquids. The most commonly used supercritical fluid in extraction is carbon dioxide (CO2) due to its favorable properties. When CO2 is exposed to high pressure and temperature, it becomes a supercritical fluid with properties such as:
- Density: Supercritical fluids possess higher densities than gases, which enhances their solvating power and improves mass transfer during extraction.
- Viscosity: The viscosity of supercritical fluids is similar to that of gases, allowing for easier penetration into the matrix of the material being extracted.
- Diffusivity: Supercritical fluids exhibit higher diffusivity than liquids, enabling efficient transport of solutes within the extraction system.
The CO2 Extraction Process
The principle of supercritical fluid extraction involves utilizing the solvating power of supercritical fluids to dissolve and extract target compounds from solid or liquid matrices. The extraction process typically follows these key steps:
- System Setup: The extraction system consists of a high-pressure extraction vessel, a pump to compress and circulate the supercritical fluid, and a separator to collect the extracted compounds.
- Loading the Material: The target material, such as plant matter or a solid sample, is placed inside the extraction vessel.
- Compression: The supercritical fluid, often CO2, is pressurized to a level above its critical pressure and heated to a temperature above its critical temperature. This transforms the CO2 into a supercritical state.
- Solvation and Extraction: The supercritical fluid is introduced into the extraction vessel, where it permeates the matrix and dissolves the target compounds. The solubility of the compounds in the supercritical fluid depends on factors such as temperature, pressure, and the nature of the target compounds.
- Separation: The mixture of supercritical fluid and dissolved compounds is directed to a separator where the pressure is reduced. As the pressure decreases, the supercritical fluid reverts to its gaseous state, leaving behind the extracted compounds in a concentrated form.
4 Advantages of Supercritical Fluid Extraction
Supercritical fluid extraction offers several advantages over traditional extraction methods. These include:
- Selectivity: The tunable properties of supercritical fluids allow for selective extraction of target compounds by adjusting temperature, pressure, and other parameters. This enables the extraction of specific compounds while leaving undesirable components behind.
- Mild and Green Process: Supercritical fluid extraction typically operates at lower temperatures compared to other methods, reducing the degradation of heat-sensitive compounds. Additionally, it eliminates the need for organic solvents, making it an environmentally friendly extraction technique.
- High Efficiency: Supercritical fluids have excellent mass transfer properties, resulting in efficient extraction with shorter extraction times and higher yields.
- Versatility: Supercritical fluid extraction can be applied to various industries, including food, pharmaceuticals, cosmetics, and natural product extraction. It can extract a wide range of compounds, from essential oils and flavors to pharmaceutical actives.
Conclusion
The principle of supercritical fluid extraction lies in harnessing the unique properties of supercritical fluids to efficiently extract desired compounds from various materials. By leveraging the density, viscosity, and solvating power of supercritical fluids, this technique offers a selective, efficient, and environmentally friendly alternative to conventional extraction methods. With its versatility and potential for customization, supercritical fluid extraction continues to find applications in numerous industries, driving advancements in extraction processes and opening new possibilities for obtaining valuable compounds from diverse sources.
What is supercritical fluid extraction
Supercritical fluid extraction (SFE) is the process of separating one component (the extractant) from another (the matrix) using supercritical fluids as the extracting solvent.
Supercritical CO2 extraction (SCFE) is used particularly in the food, beverage, cosmetics and pharmaceutical industry for extracting natural substances, aromas, fats, oils, waxes, polymers, enzymes and colourants in their supercritical physical state.
CO2 is a natural and environmentally-friendly solvent which has advantages over synthetic and harmful media such as n-hexane when it comes to sustainability.
Supercritical CO2 extraction machine