Essential oils
What is essential oils?
Essential oils are compounds extracted from plants, rather than being synthetically manufactured in labs, essential oils are extracted from plant materials through removal methods that are suited to the specific plant part containing the oils.
The oils capture the plant’s scent and flavor, or “essence.”, unique aromatic compounds ( terpene compounds) give each essential oil its characteristic essence.
Notes: The way the oils are made is important, as essential oils obtained through chemical processes are not considered true essential oils.
What compounds does essential oil contain?
The essential oil is highly valued as it’s believed to be the liquid representation of its source. Each essential oil’s chemical makeup defines the smell and any associated interactions with the body.
Without getting too complicated, essential oils are mostly made up of compounds like alcohols; ketones; phenols; oxides, and hydrocarbons like terpenes.
What are terpene compounds?
Terpenes are organic compounds that give smells to trees, plants, herbs, fruits, and vegetables. Terpenes evolved as a way for plants to protect themselves or make themselves more attractive to certain animals.
Lemongrass, for example, contains the terpene citronellal which is commonly used as a mosquito repellent.
Compared to essential oils, the extraction process is a little more complicated. While an essential oil aims to extract down the main components of a plant, a terpene must be completely isolated from all other substances and impurities.
More recently, terpenes have entered the public consciousness due to increased interest in their medicinal properties, such as anti-inflammation, anxiety reduction, and stress relief.
While over 20,000 different terpenes are in existence, some of the most well-known are myrcene, limonene, pinene, linalool, and beta-caryophyllene.
What’s the difference between essential oils and terpenes?
In the simplest terms, terpenes are just one part of what makes up an essential oil. Terpenes in essential oils are responsible for the smells, flavors, and sought-after medicinal effects.
To use a cooking analogy: just like tomatoes are a single ingredient in a Boeuf Bourguignon, the terpene myrcene is just a single component of juniper essential oil.
Essential oil extraction method
Essential Oils are not made, but instead, they are extracted from plant materials. Extractions are used to obtain a plant’s active botanical constituents that function as its “life force.” They are essentially the liquefied version of a plant, and they effectively allow its beneficial compounds to reach the bloodstream faster than they would by simply consuming the plant.
A herbal extract is produced when a botanical material is introduced to a solvent in which some of the plant material components dissolve. Ultimately, the solvent becomes infused with the botanical materials that it has pulled from the source plant, and this is what is referred to as the “extract.” The solution that remains at the end of the process can be liquid, or the liquid can be removed to turn the remnants of the botanical into a solid. The solvents can act as preservatives or as agents that help plant cells to break down and release their contents.
How are essential oils extracted?
Popular extraction methods include:
- Steam Distillation
- Solvent Extraction
- CO2 Extraction
- Maceration
- Enfleurage
- Cold Press Extraction
- Water Distillation
The method of extraction affects essential oil quality by way of pressure and temperatures applied.
Some extraction methods are optimization suited to particular plant types and parts; for example, Cold Press extraction is better than Enfleurage for obtaining oils from citrus fruit peels, because the peels need to be pierced and squeezed, which is not achievable through Enfleurage.
Steam Distillation
- Distillation is a method of separating components based on differences in volatile constituents in a heated mixture.
- Steam distillation involves bubbling steam through the plant material.
- The temperature of the steam is easy to control, making it ideal for heat-sensitive essential oils.
- The essential oils contained in plants are immiscible in water and have a higher boiling point, allowing the essential oil to vaporize at a lower temperature than it normally would on its own.
Essential oils are produced in the cells of aromatic plants and are held in specialized glands. They are released from the plant and collected (concentrated) most often through steam distillation (and sometimes hydro or water distillation or a combination thereof).
Steam Distillation is the most popular method used to extract and isolate essential oils from plants for use in natural products. This happens when the steam vaporizes the plant material’s volatile compounds, which eventually go through a condensation and collection process.
As the distillation process is based on the difference in different physical properties such as boiling points, vapor pressure, and volatility, and then it is a physical process instead of a chemical.
Two liquids having a boiling point difference of 25 degrees Celsius or more are usually well separated by distillation. In the case of substances that are non-volatile in nature, distillation is used to separate a liquid from that compound by using the property of viscosity.
The basic principle behind it is: heating a mixture till the boiling point of volatile compounds and vapors or gas produced by this process are collected back by condensation.
Principle of Steam Distillation
When a mixture of two immiscible liquids (e.g., water and organics) is heated and agitated, the surface of each liquid exerts its own vapor pressure as though the other component of the mixture was absent. Here an individual constituent on its own extracts vapour pressure independently. The Vapour pressure of the system increases consequently.
The two immiscible liquids start to boil when the vapour pressure of these liquids outplaces the atmospheric pressure. Many organic compounds are insoluble in water. At an absolute temperature, we can purify that is below the point where these compounds decompose.
Extraction Procedure
In the extraction process, the steam passes through the organic matter that contains the compounds for separation. The steam condenses and forms a mixture of steam and matter.
This mixture gets heated further by passing more steam, which continues to pass through the matter, evaporating the mixture. Due to the reduced vapour pressure, the required organic compounds evaporate as a part of the mixture. Moreover, its extraction takes place from organic matter.
Separation Procedure
The evaporated mixture of steam and the organic compounds passes through a container that has cold water entering inside from one end. After passing the evaporated mixture through the container, it consists of cold water. This mixture passes through hot water from another end. This results in condensation of the mixture.
This collection of the mixture takes place and it settles down for separation. When the settling process occurs, the extracted organic compounds come to the top. Their separation takes place by filtering out the water from below.
THE PROCESS OF STEAM DISTILLATION TO EXTRACT ESSENTIAL OILS
A large container called a Still, which is usually made of stainless steel, containing the plant material has steam added to it.
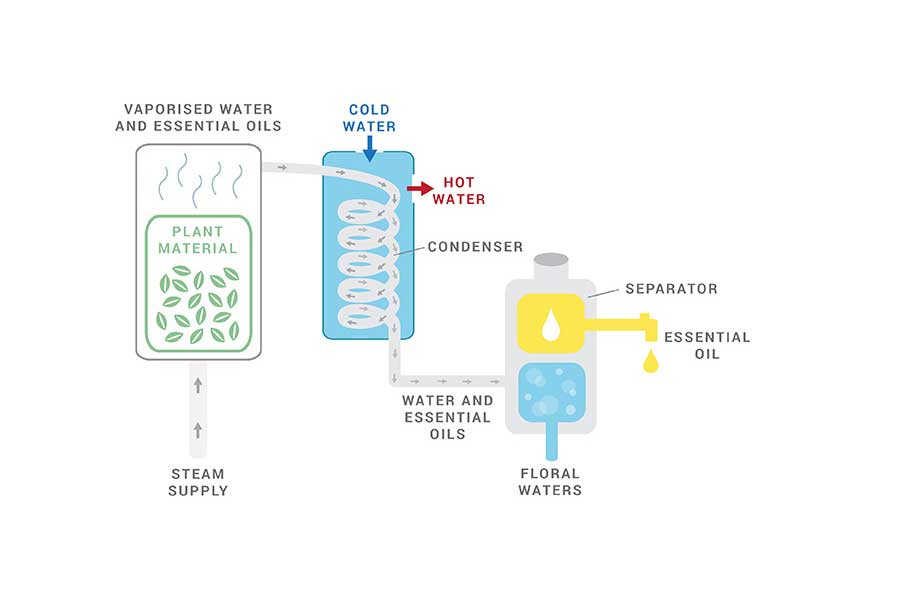
Through an inlet, steam is injected through the plant material containing the desired oils, releasing the plant’s aromatic molecules and turning them into vapor.
The vaporized plant compounds travel to the condensation flask or the Condenser. Here, two separate pipes make it possible for hot water to exit and for cold water to enter the Condenser. This makes the vapor cool back into liquid form.
The aromatic liquid by-product drops from the Condenser and collects inside a receptacle underneath it, which is called a Separator. Because water and oil do not mix, the essential oil floats on top of the water. From here, it is siphoned off. (Some essential oils are heavier than water, such as clove essential oil, so they are found at the bottom of the Separator.)
What are the advantages of steam distillation to extract essential oil?
- The method generates organic solvent-free products,
- There is no need for subsequent separation steps,
- Possesses large capacity for processing at the industrial scale,
- The equipment is inexpensive,
- Requires less fuel for the steam boiler for extraction of oils
Quality Control
People or companies who wish to use steam distillation to extract essential oils might have concerns about the quality of the extract they produce if they plan to use the extract for aromatherapy or medicinal purposes. Steam distillation allows you to control the temperature and amount of steam you apply to the plant material. Keeping the temperature right at the boiling point of water allows for the least amount of degradation to delicate essential oils.
Wide Application
Steam distillation is useful for extracting most fats, oils, and waxes.
This process works well for types of substances that do not mix with water, which are known as immiscible substances.
Steam distillation is useful for extracting most fats, oils, and waxes. This process works well for types of substances that do not mix with water, which are known as immiscible substances. Because of its wide application to this kind of material, steam distillation can be a cost-effective method to invest in to extract a diverse array of immiscible substances. Also, because the steam temperature can remain at the boiling point of water, this process has a cost-benefit of requiring less fuel for the steam boiler.
Relatively Cheap Price
Compared with CO2 extraction, the cost of steam distillation equipment is lower a lot. In most cases, steam distillation can satisfy our requirements for purity while CO2 extraction is usually used in hemp/cannabis extraction. Another important factor is that steam distillation equipment has a large capacity and is good for commercial use.
Steam Distillation VS CO2 Extraction
- Steam distillation is the most popular way to create essential oil plant extracts, but high heat from steam can damage the plant’s chemical profile and steam concentrations can’t pull out the heavier plant chemicals like cannabinoids.
- Steam distillation has a lower oil yield and does not trap most biochemical compounds as revealed in the GCMS result obtained from the samples.
- Steam distillations cause sacs in plant material to open up and release their oils, but unfortunately, this process can’t extract heavier compounds.
- The oil that is released by the steam’s heat is then carried into the condenser where the steam is turned into water and the oil separates out. This method can also damage volatile light terpenes due to the high heat. Vacuum distillation allows for steam distillation at lower temperatures, but the equipment is more expensive.
- CO2’s low supercritical temperature preserves the whole plant extract and can even pull out the heavy medicinal molecules like cannabinoids and carotenoids.
- CO2 extracts are also 100% pure. For many plants, CO2 extracts prove to create superior dietary and medicinal supplements.
- CO2 creates superior oils for plants that have heavier medicinal molecules such as frankincense and cannabis oil.
Solvent extraction
Another popular essential oil extraction method is solvent extraction. This modern method implements food-grade solvents such as ethanol, benzene, dimethyl, or hexane to isolate the oils. It is optimization suited for plant materials that yield low amounts of essential oil, that are largely resinous, or that are delicate aromatics unable to withstand the pressure and distress of steam distillation.
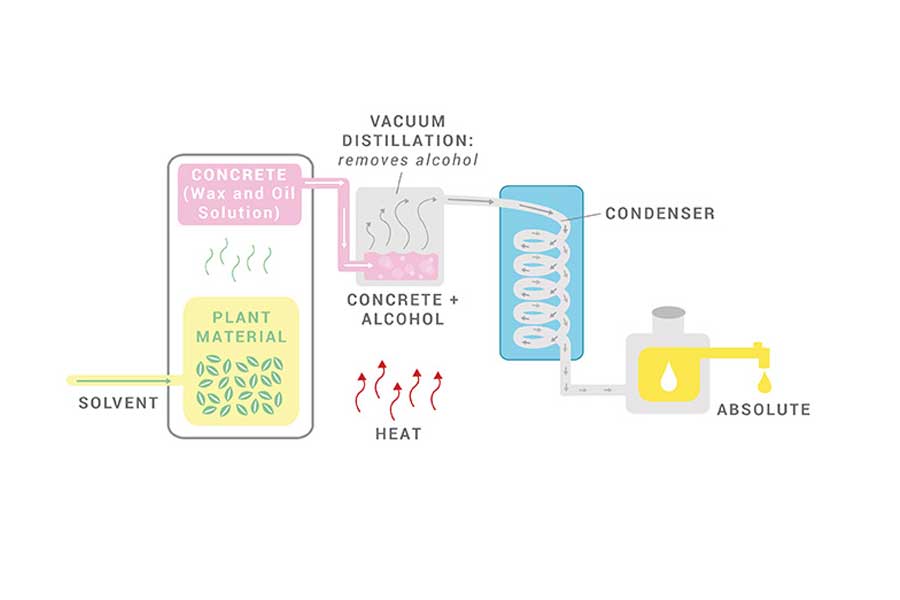
Solvent extraction is often used for extracting essential oils from delicate aromatics, such as jasmine or rose, which are generally unable to withstand the pressure of steam distillation. Plus, solvent extraction typically produces a finer fragrance than most other extraction methods which further adds to its appeal for certain applications.
Through this process, the non-volatile plant material such as waxes and pigments, are also extracted and sometimes removed through other processes.
During solvent extraction, the plant materials are covered by a solvent and dissolved into it. Once the solvent absorbs the essential oil, the resulting extract is evaporated so only the plant oil is left behind. Technically, the remaining oil is known as an absolute, not an essential oil. An absolute is a highly concentrated aromatic substance that closely resembles the plant’s natural aroma. Additionally, it has a more vivid color than essential oil.
As such, solvent extraction is often used to create extracts for perfume or cosmetic applications. The main downside to this type of extraction method is the risk that trace amounts of the solvent may not get fully evaporated. As such, small quantities of harsh chemicals may remain in the final absolute which could cause irritation when used.
Solvent extraction vs CO2 extraction
Solvent extraction
Solvent and CO2 extraction are used to extract lipid or oil-soluble nutrients from plant material. Solvent extraction uses volatile synthetic compounds such as ethyl acetate, diethyl ether, dichloromethane, and chloroform to solubilize plant components such as essential oils to remove them from the plant’s structure. The solvent is then vacuum evaporated leaving the essential oil. Most essential oils called absolutes are created from solvent extraction.
Disadvantages of solvent extraction
- Butane and Ethanol are flammable
- As well as destroying plant waxes, this form of extraction may produce oil that contains chlorophyll or other harmful contaminants.
Why is SCF CO2 better than Ethanol Extraction?
CO2 extraction
CO2 extraction uses liquid carbon dioxide (CO2) to solubilize fats and oils from the plant structure. It is believed that this extraction method gives a product that is botanically identical to what is in the plant when compared to steam distillation and solvent extraction products of the same plant material.
There are some vegetable/seed oils such as rosehip oil that are marketed as a better product compared to cold pressing (which is the standard practice). CO2 oils do have greater fat content and higher peroxide content compared to cold pressing. This is a good example of not understanding an extraction technique or what is produced.
Disadvantages of CO2 extraction
- Expensive: You can only complete the process with the aid of extremely expensive equipment.
- Technical Ability: CO2 extraction is not something that should be attempted by an amateur chemist.
Further reading
How Does Extraction Compare to Distillation?
The biggest difference between these two processes is this:
Solvent extraction: It purifies a substance whether it is in its liquid or solid phase
Solvent distillation: It purifies a substance only in its liquid phase
Applications of Distillation and Extraction
Distillation is commonly used in the petroleum industry and in the solvent industry. For instance, distillation is the favored method to separate acetic acid from acetone, benzene from toluene, and methanol and ethanol from water.
Extraction is commonly used in industries such as pharmaceuticals, fragrances, essential oils, and food products. It is the favored method that can safely isolate organic compounds such as nitrated aromatic compounds, phenol, and aniline from water.
Types of Solvent Extraction & Distillation
When it comes to the types of distillation or extraction used, there are many from vacuum to steam. However, the main difference between these types is the way compounds are separated from one another.
Insolvent distillation, you have these types of techniques:
- Vacuum distillation
- Simple distillation
- Zone distillation
- Steam distillation
- Fractional distillation
- Air-sensitive vacuum distillation
- Short path distillation
The most commonly used form of distillation is a simple distillation and fractional distillation. The simple distillation process is used when two liquids have different boiling points whereas fractional distillation is used when two liquids have equivalent boiling points.
In the solvent extraction process, the most common types are:
Solid-liquid extraction- involves isolating a substance from a solid using a fluid solvent.
Liquid-liquid extraction is used to separate a substance from a liquid.
supercritical CO2 extraction
This form of essential oils extraction is actually divided into supercritical, subcritical, and ‘mid-critical’ categories. But supercritical is by far the most commonly used. In fact, it is the most regularly used extraction method of all because it is safe and provides a pure end product.
Essential oils derived from the supercritical CO2 extraction of herbs are similar to the oils produced through distillation in that they can be used in aromatherapy and natural perfumery.
Oils derived from steam distillation vary in their qualities depending on the temperatures, pressures, and length of time applied for the process. The CO2 extraction process might thus produce higher quality oils that have not been altered by the application of high heat, unlike the steam distillation process. In CO2 extraction, none of the constituents of the oil are damaged by heat.
In simple terms, CO2 extraction uses pressurized carbon dioxide (CO2) to pull essential oils (and other phytochemicals) from the plant. CO2 acts like a solvent at certain temperatures but possesses none of the dangers. While it is safe and effective, it also involves expensive equipment which freezes the CO2 gas and compresses it into a supercritical cold liquid state.
The difference between traditional distillation and supercritical CO2 extraction
Thus, the difference between traditional distillation and supercritical extraction is that instead of heated water or steam, CO2 is used as a solvent in the latter method. The supercritical extraction process operates at temperatures between 95 to 100 degrees F whereas steam distillation operates at temperatures between 140 to 212 degrees F.
In steam distillation, the molecular composition of both the plant matter and the essential oil is changed due to the temperature applied. On the other hand, a CO2 extract is closer in chemical composition to the original plant from which it is derived, as it contains a wider range of the plant’s constituents.
For example, CO2 Extraction of German Chamomile flowers yields a green extract, because the absence of heat means it was not altered from its natural state or “denatured.” The resulting extract is thus more similar in composition to the original flower than the distilled essential oils.
CO2 extracts are usually thicker than their essential oil counterparts and often give off more of the aroma of the natural herb, spice, or plant than a distilled essential oil. CO2 extracts have been said to contain more plant constituents than the amount extracted from the same plant using steam distillation.
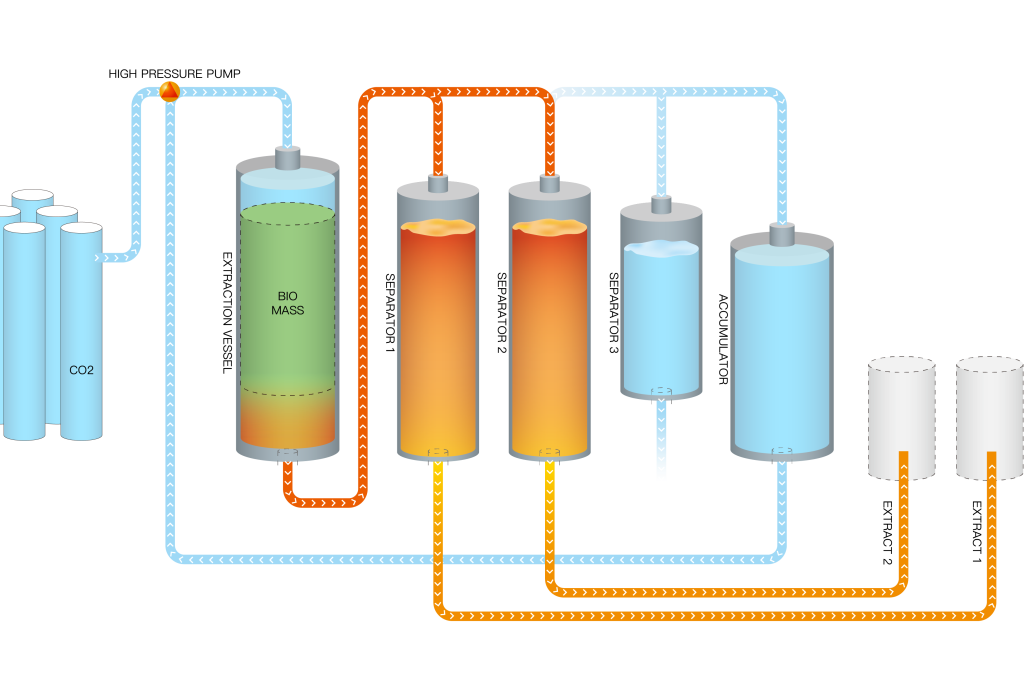
THE CO2 EXTRACTION PROCESS
CO2 typically behaves like a gas at standard pressure and temperature. And it is easily changed to a solid while in this state. The solid version of the gas is known as dry ice. Using the aforementioned equipment in a lab, you can turn CO2 gas into a liquid by ensuring the temperature drops below -69 degrees Fahrenheit while increasing the pressure to over 75 pounds per square inch (psi).
Once you have the liquid CO2, the next step is to increase the temperature and pressure past the point where the liquid becomes ‘supercritical.’ This term means the CO2 can now adopt properties halfway between a gas and liquid simultaneously. Effectively, the supercritical CO2 can fill a container (like gas) while also maintaining density (like a liquid). When CO2 is in its supercritical state, it is ideal for chemical extraction. In this state, it is pumped into a chamber filled with plant matter.
Because of the liquid properties of the gas, the CO2 functions as a solvent on the natural plant matter, pulling the oils and other substances such as pigment and resin from the plant matter. The essential oil content then dissolves into the liquid CO2.
The CO2 is brought back to natural pressure and evaporates back into its gaseous state, while what is left is the resulting oil.
CO2 is colorless, odorless, and can be easily and completely removed by releasing the pressure in the extraction chamber. It is what we exhale and is needed by plants in order for them to thrive, which illustrates its harmlessness when employed in the extraction process. This absence of potentially harmful solvents in CO2 extraction means neither the human body nor the environment is polluted.
Maceration
Macerated oils are also referred to as infused oils. They are created when carrier oils are used as solvents to extract therapeutic properties from plant material. The benefit of a macerated oil above a distilled oil is that more of a plant’s essence is captured in the oil, because it captures heavier, larger plant molecules than the ones captured in the distillation process. This keeps the product closer to retaining more of the plant’s valuable offerings.
The ideal plant material to be infused will be harvested so that it is as dry as possible, as any plant moisture will cause the oil to become rancid and will encourage microbial growth. Adding 5% of Vitamin E oil or Wheatgerm oil (which is high in Vitamin E) will prevent rancidity.
Maceration Process
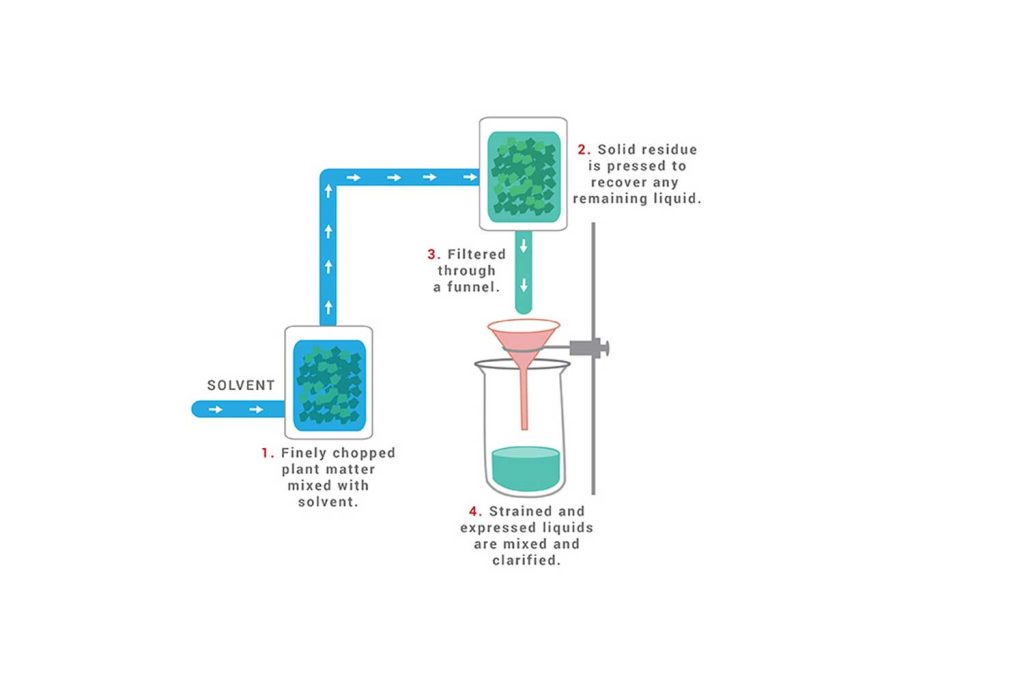
- Plant material is finely cut, crushed, or ground into a moderately coarse powder.
- Plant material is placed in a closed vessel.
- Solvent (Menstruum) is added.
- The mixture is allowed to stand for 1 week and is shaken occasionally.
- The liquid is strained.
- Solid residue (Marc) is pressed to recover any remaining liquid.
- Strained and expressed liquids are mixed.
- Liquids are clarified through filtration or subsidence.
When the maceration process is complete, the base oil will likely have changed color. The final maceration should be filtered of its plant material and poured into an airtight container to be stored in a cool, dry place for up to 12 months. A macerated oil will go cloudy or will smell bad when rancid.
5-10% of a macerated oil can be used as an ‘active botanical’ in a cosmetic formula. Used in a larger quantity, it can also replace a plain base oil.
Enfleurage
Enfleurage is not commonly used today, but it is one of the oldest methods of essential oil extraction that implements the use of fat. By the end of this process, either vegetable fat or animal fat becomes infused with the flower’s fragrance compounds. The fats that are used are odorless and solid at room temperature. The enfleurage process can be done either “hot” or “cold.” In both instances, the fat that is saturated with fragrance is called “enfleurage pomade.”
Cold Enfleurage
Highly purified and odorless vegetable or animal fat, usually lard or tallow, is spread out over glass plates in a frame called a chassis and is allowed to set.
Fresh flower petals or fresh whole flowers are then placed on top of the layer of fat and pressed in. They are allowed to set for 1-3 days or for a couple of weeks depending on the flowers that are used. During this time, their scent seeps into the fat.
The depleted petals are replaced and the process is repeated until the fat reaches the desired saturation.
The final product is the enfleurage pomade: the fat and the fragrant oil. This is washed with alcohol to separate the botanical extract from the remaining fat, which is used to make soap. When the alcohol evaporates from this mixture, the “absolute” is what is leftover.
Hot Enfleurage
The only difference in this process is that the fats are heated.
Cold-press extraction
This method is also called Expression or Scarification and is used for citrus peels in particular.
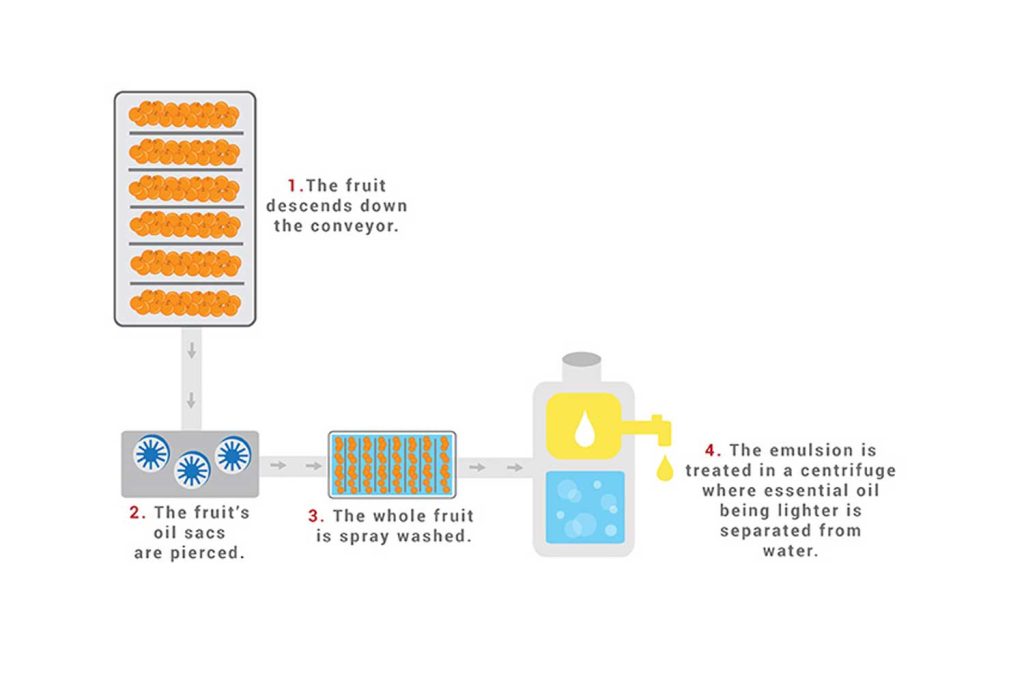
The whole fruit is placed in a device that mechanically pierces it to rupture the essential oil sacs, which are located on the underside of the rind. The essential oil and pigments run down into the device’s collection area.
The whole fruit is pressed to squeeze out the juice and the oil.
The oil and juice that are produced still contain solids from the fruits, such as the peel, and must be centrifuged to filter the solids from the liquids.
The oil separates from the juice layer and is siphoned off into another receptacle.
Water distillation
Delicate flowers such as roses and orange blossoms would clump together when introduced to steam in the distillation process, so the most effective method of extraction in this situation is to submerge fragile plant material in pure boiling water instead. The water protects the extracted oil from overheating. The condensed liquids cool down and separate from each other. The remaining water, which can sometimes be fragrant, is referred to by several names including hydrolase, hydrosol, herbal water, essential water, floral water, or herbal distillate.
Water and steam distillation
In this method that can be employed with herb and leaf material, the plant material is immersed in water in a Still to which heat is applied. Steam is fed into the main Still from outside.
So 6 Questions to Ask Before Buying Essential Oil Extraction Equipment?
The truth is that the optimization method is the one that suits both your business goals and the experience and resources available to your extractor.
For all essential oils extractors, there are a number of considerations prior to investing in any extraction equipment. Questions to consider include:
- What is your supply of starting material?
- Who are your customers?
- What is the scale at which you wish to extract?
- What is your space capable of handling in terms of size and power demands?
- What is your budget?
- How much time do you plan to spend perfecting the craft?
The answers to all of these questions will help shape the optimization extraction lab setup for your ambitions and dreams.